Long considered the “forgotten valve,” the tricuspid valve (TC) has gained increasing attention and recognition in recent years. This is due in no small part to the development of new therapeutic procedures via a transcatheter approach, which can now offer many patients an effective treatment option in addition to drug and surgical therapy.
Long considered the “forgotten valve,” the tricuspid valve (TC) has gained increasing attention and recognition in recent years. This is due in no small part to the development of new therapeutic procedures via a transcatheter approach, which can now offer many patients an effective treatment option in addition to drug and surgical therapy.
YOU CAN TAKE THE CME TEST IN OUR LEARNING PLATFORM AFTER RECOMMENDED REVIEW OF THE MATERIALS. PLEASE CLICK ON THE FOLLOWING BUTTON:
Relevant tricuspid regurgitation (TI) is found in approximately 4% of the elderly population (≥75 years of age) with a higher proportion in women [1]. Due to demographic developments, an increase in prevalence is expected in the coming years. Whereas TI was once believed to be merely a concomitant of other cardiac disease, it is now known to have a significant impact on the prognosis and clinical course of patients in its own right. Increased mortality can be seen from mild TI – even after adjusted analysis for other factors such as comorbidities, impaired left ventricular function, or pulmonary hypertension. Even after resolution of the original cause, for example, surgery or transcatheter treatment of left-sided valvular pathology, the severity of TI may persist and substantially worsen the patient’s prognosis [2,3].
Since a large proportion of patients are not eligible for open surgical treatment due to age and comorbidities and the resulting high surgical risk, and since drug therapy has significant limitations, there has been a large therapeutic gap for a long time. The goal of developing transcatheter procedures is to close this gap. The purpose of this article is to review the current understanding of the etiology, diagnostic modalities, and current interventional care options.
Etiology and clinical presentation
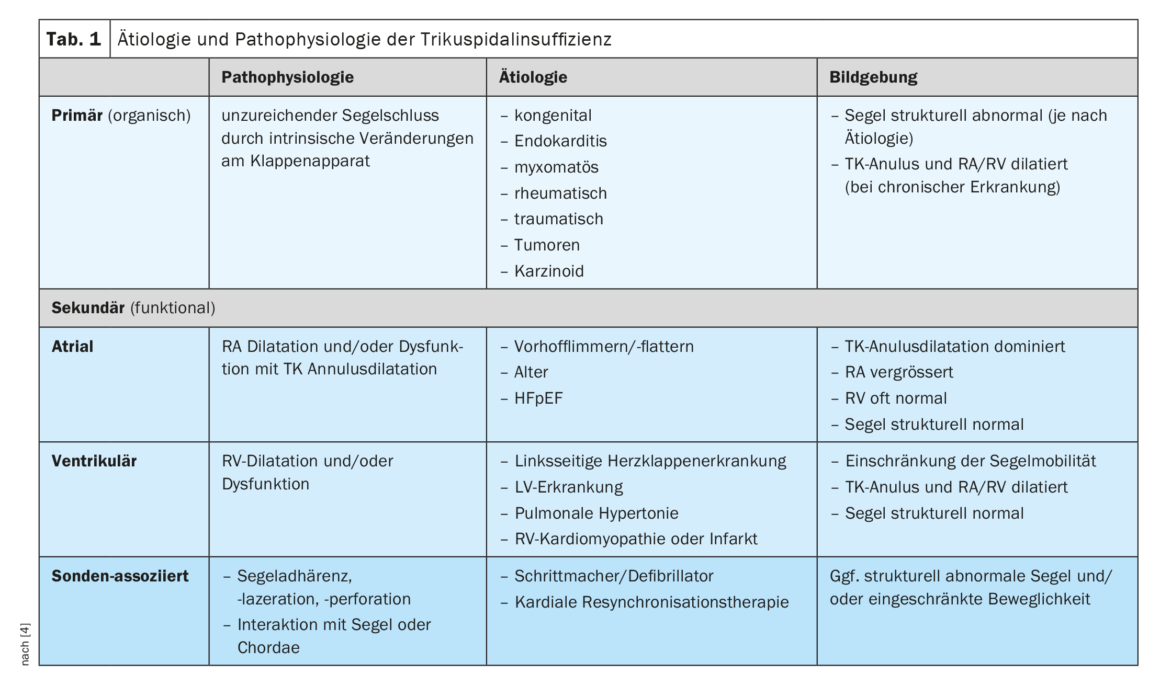
Primary (organic) TI is found in 8-10% of patients and pathophysiologically corresponds to inadequate leaflet closure due to intrinsic changes in the valve apparatus (Table 1) [4]. Etiologically, this may be due to congenital changes, endocarditis, myxomatous or rheumatic disease, trauma, tumors, or carcinoid (Hedinger syndrome). Imaging shows abnormal structure of the leaflets, depending on the underlying cause, and often subsequent dilatation of the TK annulus and right-sided cardiac cavities [5]. In contrast, in secondary (functional) atrial TI, TC annulus dilatation due to dilatation of the right atrium (RA) is the dominant mechanism. Etiologically, secondary atrial TI often results from atrial fibrillation/flutter or heart failure with preserved pump function (HFpEF). Whether TI is a consequence or a cause of VHF is the subject of current research, but it could be shown that conversion to sinus rhythm can significantly reduce the severity of TI. The most common form is secondary ventricular TI, in which RV dilatation limits or disrupts sail mobility. The underlying cause is often left-sided valvular or ventricular disease, pulmonary hypertension, or right ventricular pathology (RV, cardiomyopathy, or infarction). If impaired RV function is present, it is also a predictor of worse clinical prognosis independent of any RV dilatation [6]. Image morphologically, leaflet mobility appears to be primarily limited in systole and the right-sided cardiac cavities are dilated and/or dysfunctional. Last, TI can occur in association with a pacemaker or defibrillator probe; a form of TI that is often underestimated in its prevalence. The cause may be direct injury to the valve apparatus during implantation or mechanical interaction with a leaflet or the chordae. RV pacing-induced dysynchrony may also play a role, as the development of TI has also been observed with wireless pacemakers. Common to all forms of TI is that dilatation of the right-sided cardiac cavities and the TK annulus by the TI itself further exacerbates it, resulting in a vicious cycle of steadily increasing insufficiency.
On a clinical level, TI primarily results in symptoms of right-sided heart failure, such as peripheral edema, ascites, decreased appetite, bloating, and jugular venous congestion, due to reflux into the venous system. In very pronounced TI, marked pulsatility of the jugular vein can be observed with the naked eye during clinical examination. However, contrary to common perception, severe TI can also lead to fatigue, power intolerance, and exertional dyspnea due to forward failure (low output) . Furthermore, because of the forward failure and the TI-induced increase in central venous pressure, there is a reduction in the arterio-venous gradient in the end organs (low mean arterial pressure and high CVD). Even in subjectively symptom-free patients, this can impair liver and kidney function in particular – as cardio-hepatic or cardio-renal syndrome.
Diagnostic modalities
In addition to history and clinical signs on examination, transthoracic echocardiography (TTE) plays a crucial role in the diagnosis and evaluation of TI. However, due to various aspects, the presentation of TC and its insufficiency is very challenging, so that TI is often not recognized or underestimated. In comparison to the left side of the heart, low pressure conditions prevail at the TC and the valve structure is very delicate; in addition, the degree of severity of TI that can be depicted is strongly volume-dependent and thus subject to large fluctuations (for example, in patients on dialysis or under diuretic therapy). Additional challenges arise from the complex three-dimensional geometry of the tricuspid valve with its elliptical, nonplanar saddle-shaped annulus, often multisegmental (>3 leaflets) layout of the TC, and potential image artifacts due to pacemaker/defibrillator probes and left-sided prosthetic heart valves [7].
For diagnosis and severity, TTE is usually sufficient because the tricuspid valve is located far ventral to the thorax and close to the transducer, but transesophageal echocardiography (TEE) is usually required for accurate assessment of the underlying mechanism of TI and planning of any intervention. For special questions or the planning of certain interventions, a computed tomography (CT) or magnetic resonance imaging (MRI) may also be performed. In addition to the TI, the RV can be accurately assessed here in terms of its function and dimension. Because echocardiographic assessment of pulmonary pressure gradient in severe TI is not reliable and is often relevantly underestimated, consideration should be given to performing right heart catheterization for hemodynamic evaluation.
Various parameters are used in echocardiography to classify severity, and a distinction is usually made between mild, moderate, and severe TI. However, since, especially in the case of TC disease, patients often present or are diagnosed late in the course of the disease and the parameters are then very far above the cut-off for severe TI, experts have proposed the extension of the severity spectrum by two further levels: in addition to severe TI, there are also “massive” and “torrential” TI. (torrential) TI [8,9]. In particular, this has an impact on the appropriate interpretation of the postinterventional outcome: for example, reduction of a “torrential” TI to a severe TI may result in a marked improvement in the patient’s clinical symptoms and prognosis, even if formally echocardiographic TI remains severe. The five-stage severity classification has been used in clinical trials for several years and was also included in the recently published new ESC guidelines for valvular heart disease [10].
Therapy options
Drug: Drug treatment in TI patients is primarily based on the cardio-pulmonary comorbidities present. Treatment of heart failure or left-sided valvular heart disease with or without pulmonary hypertension should be according to current guidelines. Diuretic administration is useful to reduce symptoms, but the balancing act of optimal dosing between clinical symptoms and patients’ renal function is often difficult. Many patients already have impaired renal function at diagnosis, which may aggravate with diuretic therapy. However, even in such a case, diuretic treatment should not be completely discontinued in severe TI, as this significantly increases the risk of right-sided cardiac decompensation and also increases the renal damaging aspect of TI itself. The administration of mineralocorticoid receptor antagonists and inhibitors of the renal sodium-dependent glucose transporter SGLT2 (sodium dependent glucose co-transporter 2) may also be considered, but again, specific studies of their use in high-grade TI are lacking. The goal of drug therapy should be to achieve and maintain mild-grade TI. If this is not possible, early presentation to a heart valve center should be made, according to ESC guidelines.
Surgical: surgical treatment remains the first choice in symptomatic patients with severe primary TI. If dilatation or functional decline of the RV is already evident as a result of primary TI, surgery may also be considered in a- or oligosymptomatic patients. Surgical correction of secondary TI is most useful when a patient undergoes left-sided valve surgery. Perioperative risk probably does not increase significantly, but reduction of the TC annulus may have beneficial effects on the RV and the patient’s functional status. Isolated surgery for secondary TI, on the other hand, should be performed only in strictly selected patients who have not undergone previous cardiac surgery and in whom the disease is not advanced and RV function is intact. Two risk scores have now been developed for the assessment of operative risk in isolated TI [11,12], allowing the estimation of operative mortality and morbidity. Overall, the data on this is limited, but shows high mortality, high cost burden, long hospital stays, and protracted recovery processes in patients undergoing surgery [13,14].
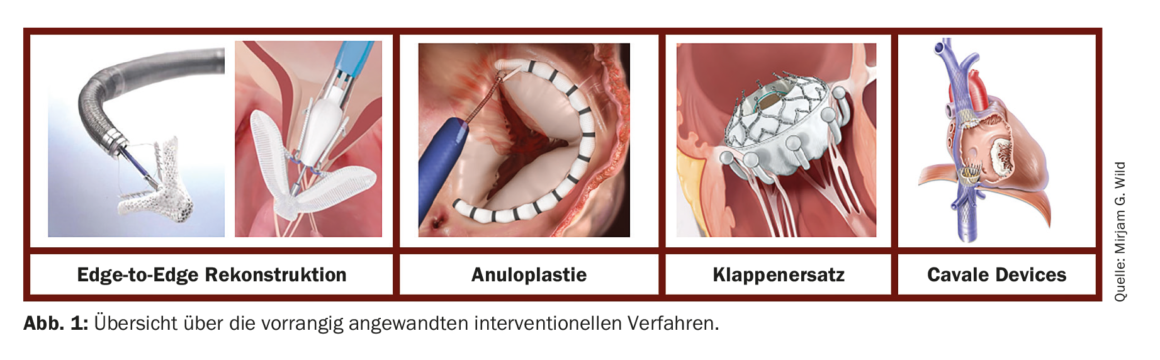
Interventional: Over the past few years, various interventional procedures have been developed for the treatment of TC via transcatheter access. Most are borrowed from surgical procedures, similar to the mitral valve. A distinction is made between reconstruction methods which aim at approximating the sails by means of a so-called edge-to-edge techniqueand those which reduce the size of the TC annulus by means of annulus refinement. In addition to reconstruction, various systems are now available for transcatheter valve replacement. Compared with mitral valve treatment, the anatomical (thinner leaflet structure, larger anular dimensions, and larger coaptation gaps) and technical circumstances (challenging imaging, more difficult navigation in the multileaflet valve apparatus) sometimes result in immense challenges, which is why interventions should only be performed at experienced and specialized centers. Figure 1 shows an overview of the procedures currently used as a matter of priority.
Most procedures use a femoro-venous access route and usually require general anesthesia because continuous TEE monitoring is required in addition to fluoroscopy. Intracardiac echocardiography ( ICE) may be useful as a complement when TEE image quality is limited.
A: Edge-to-edge reconstruction
Currently, the most commonly used transcatheter technique worldwide for the treatment of severe TI is the so-called edge-to-edge reconstruction, for which two commercially approved systems are available: the TriClip® system (Abbott Vascular, Chicago US) and the PASCAL® system (Edwards Lifesciences, Irvine US). Both systems differ in the technical characteristics of the feeding catheter, the controllability and the fine structure of the clip devices, but the basic principle is the same: at the site of greatest insufficiency jet, by bringing the valve leaflets closer together, the implant improves coaptation and thus reduces backflow. Multiple clips can be implanted in one valve and the different systems can be combined to suit the patient’s individual anatomical conditions. Both the TriClip® and PASCAL® systems have been shown in studies to be safe and beneficial [15–18]. In addition to an improvement in clinical symptoms, a reduction in right-sided cardiac dimensions, improved RV function, higher cardiac output, and a decrease in elevated liver enzymes were noted [19,20].
B: Annuloplasty
The Cardioband® system (Edwards Lifesciences, Irvine US) mimics surgical annuloplasty and was originally developed for the mitral valve. However, this system is now well established for percutaneous direct annuloplasty of the tricuspid valve and received its approval (CE Mark) in 2018 [21]. During the intervention, a ligament is anchored in the annulus by means of up to 17 screws and then tightened, which leads to a kind of tightening of the annulus and, in this way, to an improvement in sail coaptation. In a prospective study, the system achieved good technical and functional results. Due to the close proximity to the right coronary artery, its course must be closely monitored preprocedurally (by CT) and intraprocedurally (by fluoroscopy and angiography) to exclude compromise.
C: Valve replacement
Various systems are currently in preclinical and clinical trials for percutaneous replacement of the native tricuspid valve. The greatest experience and current application is for the EVOQUE system (Edwards Lifesciences, Irvine US), in which the artificial valve is stabilized by means of small anchor arms enclosing the native leaflets. The system already has some promising data [22] and CE approval is expected in the near future.
D: Cavale Devices
In patients in whom direct intervention at the tricuspid valve is not possible for anatomic reasons, upstream stent-valve systems can be implanted in the vena cavae to minimize venous return. The TricValve system (P+F PRODUCTS + FEATURES GMBH, Vienna AT) consists of two stents, each with a flap element, which are available in different sizes and can thus be adapted to the caval dimensions. The system has recently (2021) received its commercial approval based on proven safety and efficiency [23].
The currently available evidence on transcatheter therapy of TI consists largely of registry data, clinical experience reports, and prospective single-arm studies. The TriValve registry was the first large TI registry to collect multicenter data. Here, a high technical success rate as well as a low complication rate could be shown for all recorded procedures [24]. In a further comparative study (propensity-matched case-control study), 268 patients in the registry were compared with patients treated with medication alone. This demonstrated a clear survival benefit and a reduction in the re-hospitalization rate at one year [25].
Significance for everyday clinical practice
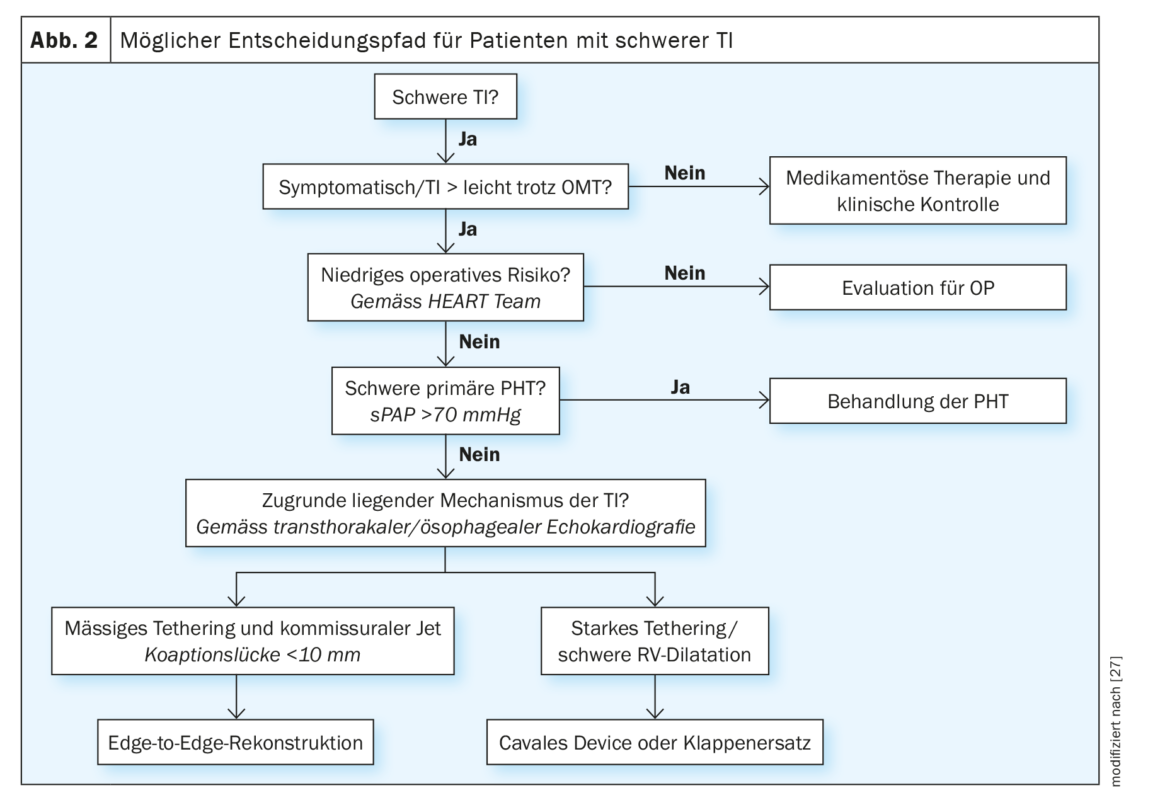
Patients with clinical or echocardiographic suspicion of relevant TI should be referred early to a specialized valve center for more detailed evaluation (according to current ESC guidelines). However, the choice of the optimal intervention time as well as appropriate treatment procedure remains a major challenge even for experts. To date, no comparative studies are available for the different transcatheter procedures, but based on clinical experience and evidence to date, orienting recommendations can be made (Fig. 2). In patients who are symptomatic despite intensified medical therapy, who have recurrent right-leading cardiac decompensations, or in whom TI cannot be reduced to a mild degree, the underlying mechanism of TI and RV function should be investigated after ruling out severe primary pulmonary hypertension. This usually includes a TEE and right heart catheterization, as well as additional CT if annuloplasty, valve replacement, or caval device is a consideration. All patients should be discussed and evaluated by an interdisciplinary Heart Team. Based on pre-procedural imaging, different anatomical characteristics can be identified that qualify the patient for the different treatment systems.
Thus, insufficiency jets with limited coaptation deficit (<8 mm) are most likely candidates for edge-to-edge reconstruction. In advanced disease with RV dilatation, large coaptation deficit, and tethering of leaflets, transcatheter reconstruction of the valve may not be technically feasible. These patients can be evaluated for orthotopic (currently only in clinical trials) or heterotopic valve replacement. The presence of a pacemaker or defibrillator probe is not a fundamental contraindication to transcatheter TI treatment. Because similar treatment outcomes were shown for this group of patients compared with patients without an electrode, the same criteria can be used to determine the treatment pathway [26]. Probe removal can also be evaluated as part of an individualized treatment plan.
Outlook
Patients with severe TI represent a very heterogeneous and complex patient population. Over the past several years, various transcatheter procedures have been developed and commercially approved for treatment, providing a therapeutic option for many previously underserved patients. Numerous studies, such as large longitudinal registries or randomized controlled trials by industry (e.g. TRILUMINATE, CLASP TR, TRISCEND II) and researchers (e.g. TRIC-I-HF) will be able to provide new insights in the coming years.
Patient selection and the choice of the appropriate treatment procedure continue to pose challenges, which is why affected patients should be referred to a specialized valve center early before the onset of RV dysfunction. Likewise, a more comprehensive understanding of the development of the disease and its underlying pathophysiological mechanisms is to be strived for, in order to be able to modulate the disease already in its course.
Take-Home Messages
- Tricuspid regurgitation (TI), even independent of other factors, has a significant impact on the prognosis and clinical course of patients.
- New transcatheter procedures such as edge-to-edge reconstruction or
valve replacement offer alternative treatment options for many previously underserved patients. - Affected patients should be referred to a specialized valve center at an early stage so that a heart team can determine the indication,
the timing and appropriate treatment procedure can be identified. - Auf Basis des derzeitigen Wissensstandes kann eine interventionelle
Therapie der TI bei den folgenden Konstellationen erwogen werden:- Symptomatic, severe TI,
- Symptomatic, moderate-to-severe TI with previous right-leading cardiac decompensations,
- Symptomatic, moderate-to-severe TI with increasing diuretic doses.
Literature:
- Topilsky Y, Maltais S, Medina Inojosa J, et al.: Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovascular imaging 2019; 12: 433–442.
- Nath J, Foster E, Heidenreich PA: Impact of tricuspid regurgitation on long-term survival. Journal of the American College of Cardiology 2004; 43: 405–409.
- Wang N, Fulcher J, Abeysuriya N, et al.: Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. European heart journal 2019; 40: 476–484.
- Praz F, Muraru D, Kreidel F, et al.: Transcatheter treatment for tricuspid valve disease. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2021; 17: 791–808.
- Hahn RT, Waxman AB, Denti P, Delhaas T: Anatomic Relationship of the Complex Tricuspid Valve, Right Ventricle, and Pulmonary Vasculature: A Review. JAMA cardiology 2019; 4: 478–487.
- Dietz MF, Prihadi EA, van der Bijl P, et al.: Prognostic Implications of Right Ventricular Remodeling and Function in Patients With Significant Secondary Tricuspid Regurgitation. Circulation 2019; 140: 836–845.
- Hahn RT, Thomas JD, Khalique OK, et al.: Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovascular imaging 2019; 12: 469–490.
- Hahn RT, Zamorano JL: The need for a new tricuspid regurgitation grading scheme. European heart journal cardiovascular Imaging 2017; 18: 1342–1343.
- Go YY, Dulgheru R, Lancellotti P: The Conundrum of Tricuspid Regurgitation Grading. Frontiers in cardiovascular medicine 2018; 5: 164.
- Vahanian A, Beyersdorf F, Praz F, et al.: 2021 ESC/EACTS Guidelines for the management of valvular heart disease. European heart journal 2022; 43: 561–632.
- Dreyfus J, Audureau E, Bohbot Y, et al.: TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. European heart journal 2022; 43: 654–662.
- LaPar DJ, Likosky DS, Zhang M, et al.: Development of a Risk Prediction Model and Clinical Risk Score for Isolated Tricuspid Valve Surgery. The Annals of thoracic surgery 2018; 106: 129-136.
- Zack CJ, Fender EA, Chandrashekar P, et al.: National Trends and Outcomes in Isolated Tricuspid Valve Surgery. Journal of the American College of Cardiology 2017; 70: 2953–2960.
- Alqahtani F, Berzingi CO, Aljohani S, et al.: Contemporary Trends in the Use and Outcomes of Surgical Treatment of Tricuspid Regurgitation. Journal of the American Heart Association 2017; 6.
- Braun D, Nabauer M, Orban M, et al.: One-year results of transcatheter treatment of severe tricuspid regurgitation using the edge-to-edge repair technique. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2018; 14: e413–e415.
- 16 Nickenig G, Weber M, Lurz P, et al: Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2019; 394: 2002-2011.
- Kodali S, Hahn RT, Eleid MF, et al.: Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. Journal of the American College of Cardiology 2021; 77: 345–356.
- Wild MG, Low K, Rosch S, et al.: Multicenter Experience With the Transcatheter Leaflet Repair System for Symptomatic Tricuspid Regurgitation. JACC Cardiovascular interventions 2022; 15: 1352–1363.
- Karam N, Braun D, Mehr M, et al.: Impact of Transcatheter Tricuspid Valve Repair for Severe Tricuspid Regurgitation on Kidney and Liver Function. JACC Cardiovascular interventions 2019; 12: 1413–1420.
- Orban M, Braun D, Deseive S, et al.: Transcatheter Edge-to-Edge Repair for Tricuspid Regurgitation Is Associated With Right Ventricular Reverse Remodeling in Patients With Right-Sided Heart Failure. JACC Cardiovascular imaging 2019.
- Nickenig G, Weber M, Schuler R, et al.: Two-year Outcomes with the Cardioband Tricuspid System from the Multicentre, Prospective TRI-REPAIR Study. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2020.
- Webb JG, Chuang AM, Meier D, et al.: Transcatheter Tricuspid Valve Replacement With the EVOQUE System: 1-Year Outcomes of a Multicenter, First-in-Human Experience. JACC Cardiovascular interventions 2022; 15: 481–491.
- Estevez-Loureiro R, Sanchez-Recalde A, Amat-Santos IJ, et al.: 6-Month Outcomes of the TricValve System in Patients With Tricuspid Regurgitation: The TRICUS EURO Study. JACC Cardiovascular interventions 2022; 15: 1366–1377.
- Mehr M, Taramasso M, Besler C, et al.: 1-Year Outcomes After Edge-to-Edge Valve Repair for Symptomatic Tricuspid Regurgitation: Results From the TriValve Registry. JACC Cardiovascular interventions 2019; 12: 1451–1461.
- Taramasso M, Benfari G, van der Bijl P, et al.: Transcatheter versus medical treatment of symptomatic severe tricuspid regurgitation. Journal of the American College of Cardiology 2019.
- Taramasso M, Gavazzoni M, Pozzoli A, et al.: Outcomes of TTVI in Patients With Pacemaker or Defibrillator Leads: Data From the TriValve Registry. JACC Cardiovascular interventions 2020.
- Winkel MG, Brugger N, Khalique OK, et al.: Imaging and Patient Selection for Transcatheter Tricuspid Valve Interventions. Frontiers in cardiovascular medicine 2020; 7: 60.
HAUSARZT PRAXIS 2023; 18(4): 8–13

![Dapagliflozin nun auch bei erhaltener Auswurffraktion zugelassen [1,4]**](https://myhealthplatform.ch/wp-content/uploads/2023/06/herz_tablet_istock-1206797366-500x383.png)
Leave A Comment
You must be logged in to post a comment.